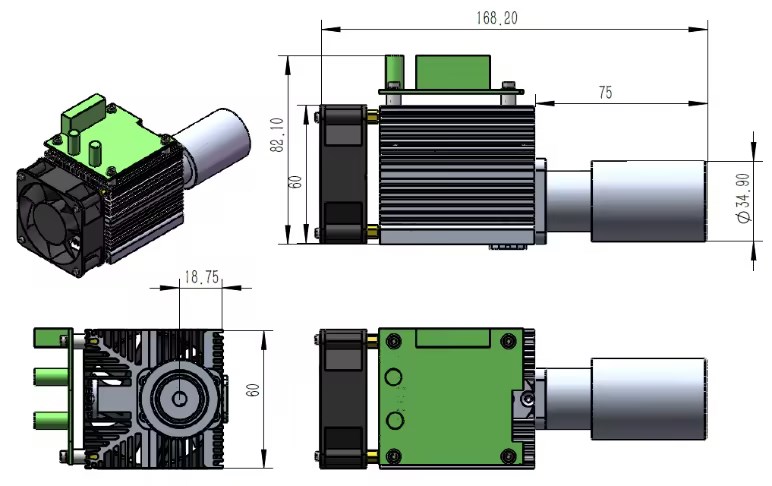
Product Description
When the molten elemental silicon solidifies under supercooling conditions, the silicon atoms are arranged into many crystal nuclei in the form of a diamond lattice, and if these crystal nuclei grow into grains with different crystal surface orientations, these crystalline grains are combined to crystallize into polysilicon.
Main Advantages:
Polycrystalline silicon has gray metallic luster and density 2.32~2.34g/cm3. Melting point 1410 °c. Boiling point 2355 °c. Soluble in mixed acids of hydrofluoric acid and nitric acid, insoluble in water, nitric acid and hydrochloric acid. The hardness is between germanium and quartz, brittle at room temperature, and easy to break when cutting. Heating to above 800 °C is ductile, 1300 °C shows obvious deformation. It is inactive at room temperature, and reacts with oxygen, nitrogen, sulfur, etc. at high temperature. In the state of high temperature melting, it has great chemical activity and can interact with almost any material. It has semiconductor properties and is an extremely important excellent semiconductor material, but trace impurities can greatly affect its conductivity. The electronic industry is widely used in the manufacture of semiconductor radios, tape recorders, refrigerators, color TVs, video recorders, electronic computers and other basic materials. It is obtained by chlorination of dried silica powder and dry hydrogen chloride gas under certain conditions, and then condensation, rectification and reduction.
Typical applications:
Polysilicon is a direct raw material for the production of monocrystalline silicon, and is the basic material of electronic information for contemporary artificial intelligence, automatic control, information processing, photoelectric conversion and other semiconductor devices.
Material Properties
| Material | Polycrystalline Si |
| Chemical Formula | Si |
| Density | 2.33g/cm³ |
| Purity | 99.999% |
| Dimension tolerance | ±0.1mm |